I’m finding that I’m becoming increasingly fascinated by shape. It seems such a simple thing yet scratch the surface only a little and the complexity comes pouring out. Take simple tiling problems; I can tile my floor with squares or regular hexagons, but not regular octagons - they’ll always leave annoying gaps. From a statistical mechanics point of view those gaps are very important, little sources of entropy that you can’t get rid of. In three dimensions understanding the packing of tetrahedra has proved no simple task. But that’s a story for another day.
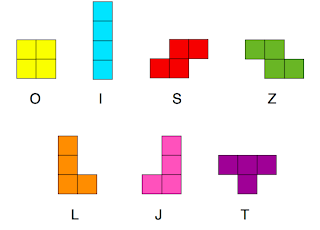
So it came as no surprise that I was very taken with Lev Gelb’s talk on polyominoes at the Brno conference. Polyominoes are connected shapes on a two dimensional lattice. A monomino is a square, a domino you know. Tetrominoes are made of four squares and are exactly like the pieces from Tetris. Assuming that they’re stuck in the plane (so you can’t flip them over) there are 7 tetrominoes.
All of these can individually tile space so it is possible to have no gaps. Shapes such as S and Z, L and J are chiral opposites. There are some subtle differences in the rotational entropy as well. O has a single rotational state. I, S and Z have 2, the rest have 4.
Many have studied these sorts of shapes before. Lev’s group are looking at it in the context of nanoparticles adsorbing to a surface. What they’ve done is to run really efficient simulations in an open system (grand canonical). They fix a chemical potential - this is kind of like fixing the pressure - and then measure what how many particles are on the surface.
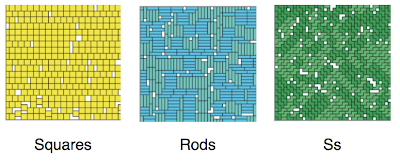
At low pressure the density you get is the same for all shapes, it’s too low to notice the difference. At high pressures you start to see the difference. As you may expect, with their simpler shape, the squares are the easiest to pack in. For the same pressure you get the highest density of all the shapes. The pictures below (from the paper) show what the high density configurations look like.
You can see there’s quite a bit of ordering. But is it long range ordering, i.e. a phase transition? The quick answer is no. The correlations build up, and in interesting ways unique to each shape, but there are no discontinuities that could be considered a proper phase transition. It would seem you need more than four monomers for that. How many more? Well just one apparently! With pentominoes you do see phase transitions with some shapes.
On a related note you can ask, how long must the rods be before they will form a liquid crystal? For that they must be seven squares long (according to JCP 128, 214902).
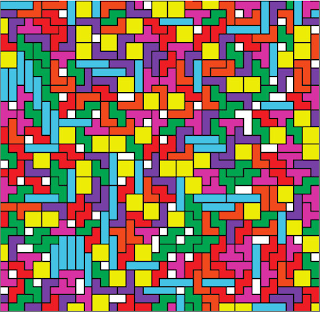
Finally they move on to mixtures. There a lots of combinations you can do. A nice shot is the full mixture with all the shapes in.
It seems that the different shapes mix quite happily, they don’t split into domains of each different type. What does all this mean for tetris? Um, well it looks to me that if they mix pretty well then it should be possible to play a perfect game! OK, I’m clutching at straws a bit there.
I could geek out about shapes all day. See the detailed paper (open access) at Langmuir, 2009, 25 (12), pp 6702–6716. Or Lev’s group pages for some nice animations.